Abstract
Introduction: While AML is phenotypically diverse, genome-wide sequencing studies indicate that the median number of non-synonymous mutations is eight (Science 339,1546, 2013) and that the same pathways are affected in tumors with distinct genetic alterations (Nature 453,1112-1116, 2008). These insights provided the impetus to immunoaffinity purify shared antigenic moieties in human AML and examine their prevalence as well as function.
Methods: We employed a previously described method wherein immunization of rabbits with normal human peripheral blood cells whose surface charge had been modified by incubation with fluorodinitrobenzene (FDNB) elicited an antibody response that cross reacted against a broad range of human leukemias (Nature 232,197, 1971). Replicating the described methods WBCs from healthy volunteer donors were suspended at 1x107 cells per ml of PBS and mixed with equal volume of 0.86 mM FDNB for 12 to 15 minutes at room temperature. Three rabbits were immunized with FDNB-treated cells (experimental rabbits). A control rabbit was immunized with sham-treated cells (control rabbit). After complement inactivation, immune sera were absorbed against WBCs from healthy donors. Absorbed immune sera were tested in clinical AML samples by Western blotting. Immunoprecipitation of antigens from whole cell lysates of clinical AML samples was performed using IgG adsorbed on protein A/G Agarose beads. Liquid chromatography and mass spectrometry of the immunoprecipitated material was performed. Fold change in IQGAP1 expression in normal vs AML bone marrow was determined by qRT-PCR and from raw data from Gene Expression Omnibus at the NCBI. IQGAP1 expression was evaluated by immunohistochemistry in cell lines, bone marrow biopsies from AML patients and normal healthy volunteers. IQGAP1 expression was modulated by RNAi and the effect on proliferation and colony formation was measured in cell lines.
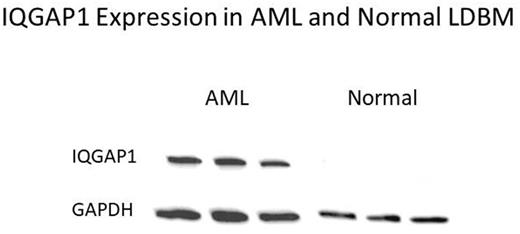
Results: Western blotting of whole cell lysates of clinical AML samples revealed bands that were recognized by immune serum from experimental rabbits but not the control rabbit. Immuno-precipitation of antigens from whole cell lysates of clinical AML samples revealed the scaffolding protein IQGAP1 as being differentially recognized in independent experiments. We focused on IQGAP1 because it regulates multiple cellular functions including signaling pathways involved in proliferation and transformation and stimulates cell motility and invasion. It is overexpressed in colon cancer, pancreatic cancer, hepatocellular carcinoma, glioma, and other cancers. (Cell Signaling 21,1471-1678, 2009). Western blots of human AML samples probed with anti-IQGAP1 antibody revealed the predicted 190 kDa band, see figure. There was 3 to 9-fold greater expression of IQGAP1 expression in AML versus normal bone marrow observed by qRT-PCR. Analysis of raw data from Gene Expression Omnibus at the NCBI revealed fold change of -3.22636, p-value 2.62 x 10e-7 between normal bone marrow versus AML. Preliminary IHC studies showed strong membranous staining in three leukemia cell lines and bone marrow biopsies from primary AML bone marrow samples but weak partial staining in normal bone marrow. Knocking down expression of IQGAP1 in K562, MV4-11 and THP1 cell lines resulted in significant decrease in proliferation and colony formation as compared to effects of scramble control. Mean and SD of colonies per 2000 cells plated were for K562: naïve 311+/-46, IQGAP1 knockdown 37+/-12, scramble control 127+/-12, MV4-11: naïve 156+/37, IQGAP1 knockdown 55+/- 8, scramble control 147+/-16, and THP1 naïve 232+/-45, IQGAP1 knockdown 17+/-2.9, scramble control 150+/-24.
Conclusions: 1. The scaffolding protein IQGAP1is overexpressed in AML compared to normal bone marrow. 2. Knocking down expression of IQGAP1 in AML cell lines resulted in significant decrease in proliferation and colony formation providing proof of concept that IQGAP1 is a potential therapeutic target in AML.
Liesveld: Onconova: Honoraria; Seattle Genetics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal